Li-Yu Lin1 and Teng-Ming Chen 1
1Department of Applied Chemistry National Chiao Tung University Hsinchu, Taiwan 300, R.O.C.
Received:
July 30, 2002
Accepted:
August 20, 2002
Publication Date:
September 1, 2002
Download Citation:
||https://doi.org/10.6180/jase.2002.5.3.07
ABSTRACT
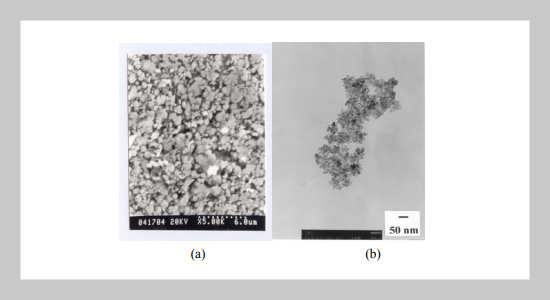
Nanoparticles of pristine and Mn2+-activated (Zn1-xCdx)S (x = 0, 0.2, 0.4, 0.6, 0.8 and 1.0) have been synthesized via a simple chemical colloid route. The size of as-prepared (Zn,Cd)S or (Zn,Cd)S:Mn2+ nanoparticles was determined to be ca. 5-10 nm in diameter with a narrow size distribution, as indicated by bright-field TEM imaging investigations. With increasing x the X-ray diffraction (XRD) peaks were found to be broadened with their intensity weakened and the corresponding diffraction angles were observed to shift toward lower 2θ for nanocrystalline (Zn1-xCdx)S:Mn2+ and (Zn1-xCdx)S phases. The λex was fount to exhibit a blue shift from 362 to 325 nm and λem was observed to exhibit also a blue shift from 460 to 425 nm, as indicated by the comparison of PLE and PL spectra for the nanocrystalline and bulk (Zn,Cd)S phases. In the nanocrystalline (Zn1-xCdx)S:Mn2+ phases the absorption of (Zn,Cd)S dominates and no absorption due to Mn2+ was observed, as indicated by the PLE spectra. On the other hand, the emission of Mn2+ dominates and λem was observed to exhibit a blue shift from 596 nm to 584 nm with x increasing from 0 to 0.8, as indicated by the PL spectra for (Zn1-xCdx)S:Mn2+ phases. The effect of synthetic routes on the PLE and PL spectra and the fluorescence decay life time for ZnS:Mn2+ has also been investigated and reported.
Keywords:
Nanoparticles, (Zn,Cd)S:Mn2+, Photoluminescence, Chemical Colloid Synthesis, Solvothermal Synthesis, TEM and SEM Imaging
REFERENCES
- [1] Goldberg, P. Luminescence of Inorganic Solids, Academic Press, N.Y., U.S.A., 1966, 207; Vij, D. R.; Singh, N., Luminescence and Related Properties of II-VI Semiconductors, Nova Science Publishers, N.Y., U.S.A., 1998, 169.
- [2] Liveri, V. T.; Rossi, M.; D'Arrigo, G. Appl. Phys. A-Mater. 1999, 69, 369.
- [3] Olshavsky, M. A.; Allcock, H. R. Chem. Mater. 1997, 9, 1367; Li, Y.; Huang, F. Z.; Zhang, Q. M. J. Mater. Sci. 2000, 35, 5933.
- [4] Patel, A. A.; Wu, F. X.; Zhang, J. Z. J. Phys. Chem. 2000, B 104, 11598.
- [5] Meulenkamp, E. A. J. Phys. Chem., 1998, B 102, 5562; Van Dijken, A.; Makkinje, J.; Meijerink, A. J. Lumin. 2001, 92, 323.
- [6] Bhargava, R. N.; Gallagher, D.; Hong, X.; Nurmikko A. Phys. Rev. Lett. 1994, 72, 416.
- [7] Bol, A. A.; Meijerink, A. Phys. Rev. 1998, B 58, R15997.
- [8] Ohde H.; Ohde M.; Bailey, F.; Kim H.; Wai C.-M. Nano Lett., in press 2002.
- [9] Hirai, T.; Sato, H.; Komasawa, I. Ind. Eng. Chem. Res. 1994, 33, 3262.
- [10] Deng, Z. X.; Wang, C.; Sun, X. M. Inorg. Chem. 2002, 41, 869.
- [11] Wang, M. W.; Sun, L. D.; Fu, X. F. Solid State Commun. 2000, 115, 493.
- [12] Saenger, D. U.; Jung, G.; Mennig, M. J Sol-Gel Sci. Tech. 1998, 13, 635.
- [13] Sun, L.; Li, Z.; Liao, C.; Yan, C. Chinese J. Luminescence 1997, 18, 280.
- [14] Rossetti, R.; Ellinson, J. E.; Gibson, J. M.; Brus, L. E. J. Chem. Phys. 1984, 80, 4464.
- [15] Alivisatos, A.; Harris, A.; Levinos, N.; Stiegerwald, M.; Brus, L. J. Chem. Phys. 1989, 89, 4001.
- [16] Eychmuller, A.; Hasselbarth, A.; Katsikas, L.; Weller, H. J. Lumin. 1991, 48-49, 745.
- [17] Chen, T.-M.; Chen, Y.-W. J. Solid State Chem. 2000, 150, 204.
- [18] Konishi, M.; Isobe, T.; Senna, M. J. Lumin. 2001, 93, 1.