Li-Yu Lin1 and Teng-Ming Chen This email address is being protected from spambots. You need JavaScript enabled to view it.1 1Department of Applied Chemistry National Chiao Tung University Hsinchu, Taiwan 300, R.O.C.
Received:
July 30, 2002
Accepted:
August 20, 2002
Publication Date:
September 1, 2002
Download Citation:
||https://doi.org/10.6180/jase.2002.5.3.07
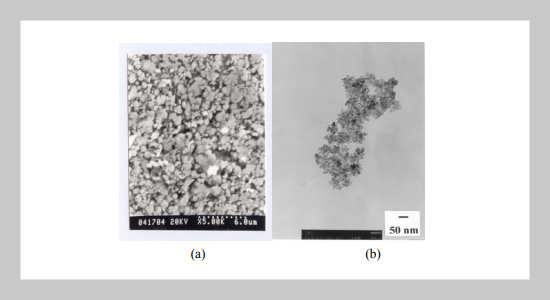
Nanoparticles of pristine and Mn2+-activated (Zn1-xCdx)S (x = 0, 0.2, 0.4, 0.6, 0.8 and 1.0) have been synthesized via a simple chemical colloid route. The size of as-prepared (Zn,Cd)S or (Zn,Cd)S:Mn2+ nanoparticles was determined to be ca. 5-10 nm in diameter with a narrow size distribution, as indicated by bright-field TEM imaging investigations. With increasing x the X-ray diffraction (XRD) peaks were found to be broadened with their intensity weakened and the corresponding diffraction angles were observed to shift toward lower 2θ for nanocrystalline (Zn1-xCdx)S:Mn2+ and (Zn1-xCdx)S phases. The λex was fount to exhibit a blue shift from 362 to 325 nm and λem was observed to exhibit also a blue shift from 460 to 425 nm, as indicated by the comparison of PLE and PL spectra for the nanocrystalline and bulk (Zn,Cd)S phases. In the nanocrystalline (Zn1-xCdx)S:Mn2+ phases the absorption of (Zn,Cd)S dominates and no absorption due to Mn2+ was observed, as indicated by the PLE spectra. On the other hand, the emission of Mn2+ dominates and λem was observed to exhibit a blue shift from 596 nm to 584 nm with x increasing from 0 to 0.8, as indicated by the PL spectra for (Zn1-xCdx)S:Mn2+ phases. The effect of synthetic routes on the PLE and PL spectra and the fluorescence decay life time for ZnS:Mn2+ has also been investigated and reported.ABSTRACT
Keywords:
Nanoparticles, (Zn,Cd)S:Mn2+, Photoluminescence, Chemical Colloid Synthesis, Solvothermal Synthesis, TEM and SEM Imaging
REFERENCES